Research
Overview
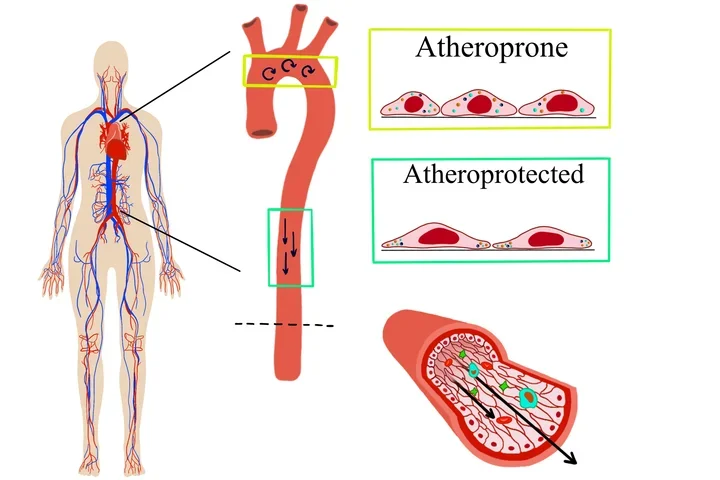
The Mack Lab studies mechanisms of endothelial cell mechanotransduction in the context of cardiovascular health and disease. We investigate how mechanical forces influence blood vessel function, both at the single cell and tissue level. We are interested in the role of blood flow forces in controlling individual endothelial cell response within a monolayer of cells. To visualize the heterogeneity of response, we utilize high resolution live cell imaging and atomic force microscopy to measure plasma membrane fluidity, quantify the dynamics of Ca2+ signaling and immune cell binding, and determine the localization of proteins at the subcellular level. In addition, we utilize mouse models to understand the role of blood flow and dyslipidemia in the development of vascular inflammation and atherosclerosis.
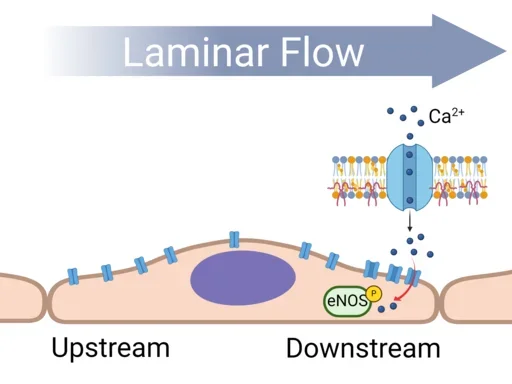
Flow-Induced Cell Polarity
Endothelial cells respond to shear stress forces on their apical surface. Such physical forces cause changes in membrane fluidity and composition. In this project we are spatially defining the ‘front’ and ‘rear’ of a flow aligned endothelial cell. We find that membrane associated proteins localize in different regions of the plasma membrane. Utilizing live cell imaging, force microscopy and fluorescent protein tracking we are investigating how flow-induced membrane polarization compartmentalizes signaling events at different locations in the cell.
Nodes of Communication within a Monolayer
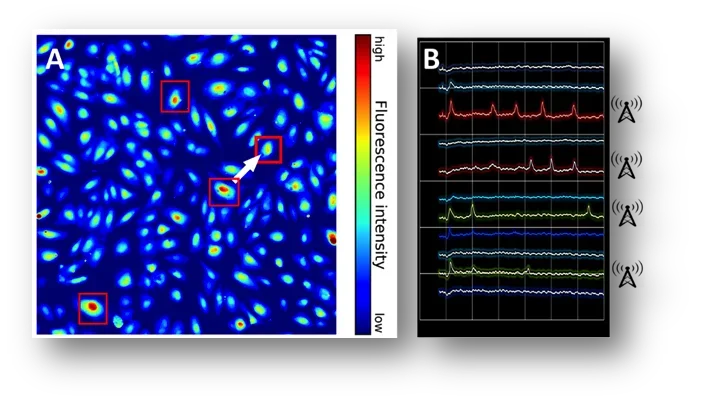
Calcium is the ultimate second messenger and involved in the activation of many signaling pathways. The endothelium converts information on shear stress intensity into changes in intracellular [Ca2+]. At the single cell level, we observe a heterogeneity of Ca2+ signaling under physiological shear stress: “responders” and “non-responders.” We are quantifying the spatial distribution and temporal patterns of “responders.” We propose that the spatial patterning of “responders” enables efficiency of signaling across the monolayer. By modifying the applied flow, we are investigating how flow (disturbed vs. laminar) affects these signaling nodes and assessing whether these nodes are associated with specific gene signaling pathways important to vascular function such as inflammation.
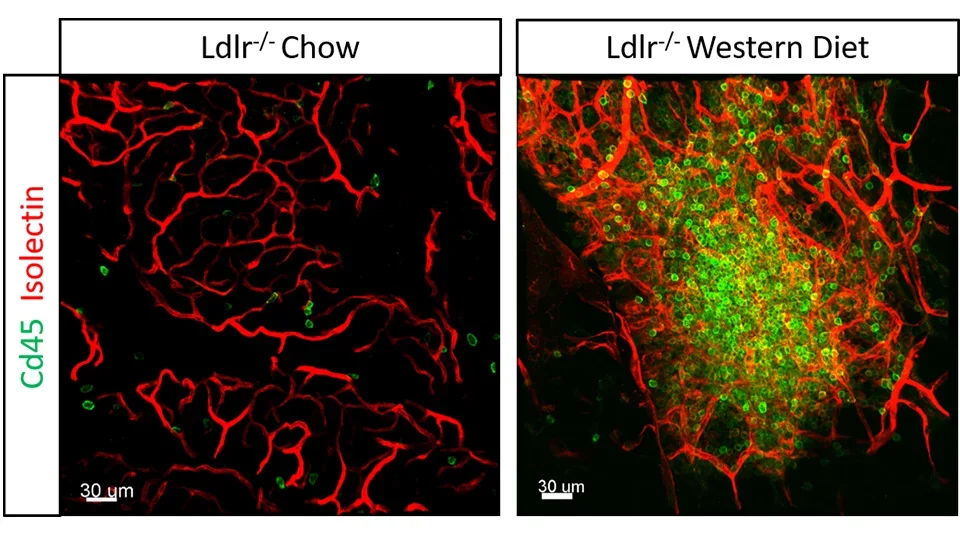
Diet Induced Vascular Inflammation
The link between diet and inflammation is an important topic in cardiovascular health. Inflammation occurs as a part of a response to harmful stimuli including high levels of circulating cholesterol, triglycerides and associated lipoproteins. In fact, it has been shown that mice fed a high fat diet have significantly higher levels of inflammatory gut bacteria and exhibit forms of vascular and neural dysfunction compared to mice fed a low fat diet. We have observed that mice with hyperlipidemia develop hyper-branched nodules of blood vessels loaded with inflammatory cells in the mesenteric tissue connected to the intestinal wall. We are currently studying the interaction of immune cells with lymphatic and blood vessels from the intestinal wall to the mesenteric tissue to understand the pathway for immune cell trafficking during diet induced inflammation.